US FDA to Review Emergent Biosolutions Nasal Spray for Drugs
2022.12.06 12:55
[ad_1]
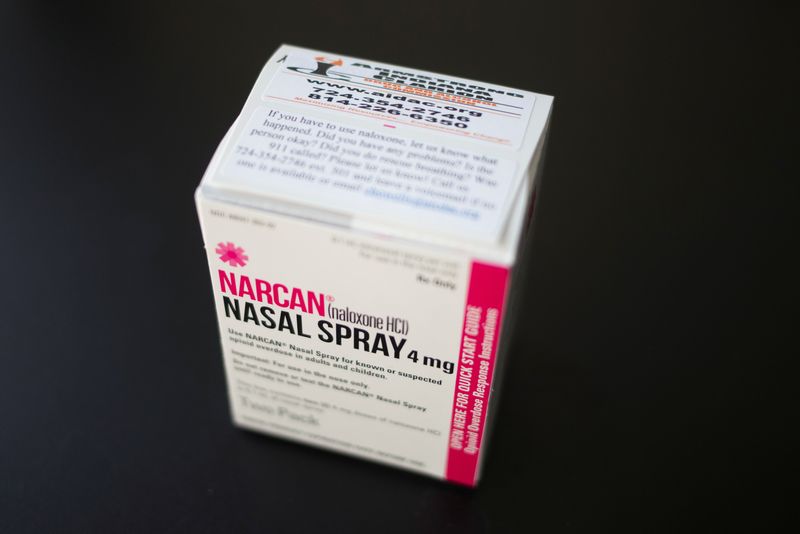
US FDA to Review Emergent Biosolutions Nasal Spray for Drugs
Budrigannews.com – Emergent Biosolutions, a contract drugmaker (NYSE:) said on Tuesday that the U.S. health regulator would prioritize reviewing its over-the-counter nasal spray as a treatment for opioid overdoses.
Emergent is requesting approval from the Food and Drug Administration of the United States for the non-prescription sale of Narcan, its nasal spray, which has already been approved for the treatment of opioid overdose in the nation.
The spray could become the first over-the-counter drug based on naloxone if it is approved by the agency by March 29.
In a note, Cowen analyst Boris Peaker stated that if Narcan is granted approval, it will likely face competition from generic versions of the drug, which will put pressure on Emergent’s margins.
Opiant Pharmaceuticals, a rival (NASDAQ:) Peaker said that Inc.’s drug nalmafene, which is also being looked at in the United States, could hurt sales of Narcan because it protects more effectively against opioid overdose.
The announcement by Emergent comes just a few weeks after the agency said that naloxone might be safe and effective enough to be sold over-the-counter in some forms, which could pave the way for its use in the federal government.
In some states, access to naloxone is restricted by legal restrictions, and even in those states, the medication is not always available to those who are most likely to overdose.
Newer strategies, such as the use of naloxone, have been developed by the administration of U.S. President Joe Biden in response to the worsening opioid crisis.
According to estimates provided by the government, there will be nearly 15% more drug-related overdose deaths in the country in 2021 than in the previous year.
According to the Maryland-based company, the data that it submitted with its application to the health regulator supports Narcan’s safe and effective use on the basis of its usability and more than five years’ worth of post-marketing studies.
The company’s shares gained nearly 3% earlier in the day before reversing course and trading down nearly 2%.








