How Cannabinoid Biotechs Are Potentially Enhancing Long-Term Stock Performance
2024.08.13 17:03
Investors in the biotechnology sector understand the familiar business model that typically includes a years long runway of proprietary drug development and clinical trials during which biotech startups might not have any revenue coming in. Many of the most-needed and well-regarded drugs on the market today were the result of this process despite the revenue delays.
However, the U.S. Food and Drug Administration (FDA) has launched four expedited approval programs to help get certain drugs on the market faster. Biotech firms working on medicinal cannabinoid drugs that know how to tap into these programs — and take the extra steps discussed below — have a chance to bring their drugs to market faster, meaning earlier revenue opportunities.
Of course, biotech stocks, including those of companies developing proprietary medicinal cannabinoid drugs, rise and fall on clinical-trial results before earnings come into the picture. However, companies who can demonstrate that their strategic approach, high quality manufacturing, pipeline, and focused implementation are on the path towards commercialization may find that they benefit from investor confidence and see their stocks re-rated higher as a result.
The FDA’s expedited approval pathways
In support of getting drugs for conditions with unmet medical needs onto the market as fast as possible, the FDA now offers four expedited approval pathways:
- Fast-track designation – expedites the development and review of new drugs or biologics to treat serious or life-threatening conditions and demonstrate the potential to address unmet medical needs.
- Breakthrough-therapy designation – expedites the development and review of drugs or serious conditions with preliminary clinical evidence suggesting the drug may offer significant improvement over available treatments.
- Accelerated approval – allows earlier approvals for drugs that treat serious conditions with unmet medical needs based on surrogate endpoints.
- Priority-review designation – FDA aims to take action on these drug applications within six months instead of the usual 10-month review period.
Biotech companies can use one or more of these programs to expedite their review process, but there’s another option that can help accelerate the clinical-trial process as well.
Repurposing previously FDA-approved drugs whose patent protection has expired, meaning they are available generically, enables biotech companies to leverage existing safety and efficacy data to reduce their expenses and the length of time needed to develop the repurposed drug. To ease the process, FDA regulations and the Hybrid Regulatory Pathway through the European Medicines Agency both allow pharmaceutical companies to submit clinical data generated previously to support their applications for a repurposed drug product.
This process typically includes repositioning drugs previously approved for other indications that have shown promise in off-label use for other conditions. Off-label can also include modifying existing drugs to provide alternate delivery systems (e.g., changing an oral medication to an IV) or adjusting its mechanism of action through extended release.
For example, Aquestive Therapeutics Inc (NASDAQ:) introduced the first and only oral, non-device, diazepam-based medication (Libervent™ Buccal Film) used for the short-term treatment of seizure clusters in children ages of two and five using its proprietary PharmFilm® technology to deliver the treatment in a new way; it is administered inside of the cheek.
Big name companies like Eli Lilly (NYSE:) and Pfizer (NYSE:) are in the drug repurposing business, too. For example, one study dubbed Pfizer’s sildenafil, better known as Viagra, as “the most successful example of a repurposed candidate drug from retrospective clinical analyses.” It was originally developed for hypertension and angina.
We could see more and more drugs repurposed in the coming years as artificial intelligence and machine learning are applied to the problem.
Creating an Attractive Market Position from Drug Repurposing in Combination
Drug repurposing can be taken a step further. Instead of repurposing a single drug, some companies are developing treatments that combine previously FDA-approved products to create new compounds.
For example, Redhill Biopharma Ltd (NASDAQ:) combined three medications (omeprazole, amoxicillin, and rifabutin) to create a new treatment for helicobacter pylori infections in adults. Additionally, Axsome Therapeutics (NASDAQ:) combined the generic medications dextromethorphan and bupropion in extended release to create a new treatment option for depression. The company also has other combination drugs in development.
Combination drugs extend patent protection and are a way to bring drugs to market more quickly. Creating or extending patent exclusivity has become particularly important to create attractive market positions for newer biopharmaceutical companies eager to reach commercialization and for big pharmaceutical companies facing massive patent cliffs with multiple blockbuster drugs losing exclusivity.
Some companies are combining existing products with new compounds such as synthetic cannabinoids to create entirely new, proprietary medications. For example, Incannex Healthcare (NASDAQ:) is working on combination cannabinoid treatments for obstructive sleep apnea (IHL-42X) and rheumatoid arthritis (IHL-675A), among other conditions in our clinical pipeline. IHL-42X is a pill for sleep apnea — a condition which lacks an FDA-approved, medicated treatment and for which the standard of care remains a CPAP machine, which has low therapeutic compliance.
The novel compound combines the FDA-approved, synthetic form of tetrahydrocannabinol (THC) known as dronabinol, which has been on the market for decades as a treatment for HIV- or AIDS-induced anorexia and/or nausea associated with chemotherapy, with the carbonic anhydrase inhibitor acetazolamide.
A Phase 2A trial revealed that IHL-42X reduced the Apnea Hypopnea Index (AHI), which measures obstructive sleep apnea, by an average more than 50% at the low dose. Additionally, 25% of patients saw their AHI decline by more than 80%. The Ph 2/3 pivotal clinical trial, REPOSA, initiated in the United States and United Kingdom in late May 2024, with early results expected in 2025.
Another company developing cannabis combination products is Tetra Bio-Pharma (TSE:TBP), which is focusing on treatments in the area of inflammation, pain, ophthalmology and oncology through a robust pipeline using multiple delivery systems. There are also other companies generally working in cannabinoids, such as Cardiol Therapeutics (NASDAQ:), which is developing one therapy for rare heart diseases and another for heart failure.
Cannabis Research at Biopharmaceutical Companies is Picking up
One of the big problems for biotech and biopharmaceutical stocks in the current market is that some smaller companies in the space are unprofitable, which is why it’s so important for these companies to get their products onto the market as quickly as possible. However, the need to ensure rigorous and proper testing of drug products can’t be understated either.
As far as ongoing cannabinoid research, the Medical Marijuana and Cannabidiol Research Expansion Act (MMCREA), approved in late 2022, was a major game changer for the U.S. pharmaceutical industry. It opened up the pathway that now allows for large-scale clinical trials of drug candidates containing cannabinoids.
Although many regulatory hurdles remain intact given that marijuana remains a Schedule I substance, companies are making progress in bringing new treatment options to market, working closely with the U.S. FDA to design studies that meet required standards and use products developed using Good Manufacturing Practices (GMP).
Investing in biotechs developing medicinal cannabinoid products
Nonetheless, the U.S. Department of Justice said in May it has proposed rescheduling marijuana, which has boosted certain cannabis exchange-traded funds (ETFs). As such, it’s no surprise that smaller biotech firms like Corbus Pharmaceuticals (NASDAQ:) and Incannex aren’t the only ones pursuing medicinal cannabinoid drug products. One big pharmaceutical company that has also been looking into the space includes Pfizer, which paid $6.7 billion to acquire clinical-stage cannabinoid firm Arena Pharmaceuticals in 2021.
Exchange-traded funds also offer exposure to a diversified portfolio of companies working on medicinal cannabinoid products. Among the highest-gaining ETFs after the DOJ news about rescheduling marijuana was the Roundhill Cannabis ETF (NYSE:), which is up 8% year to date. The AdvisorShares Pure Cannabis (NYSE:) has gained 9% year to date:
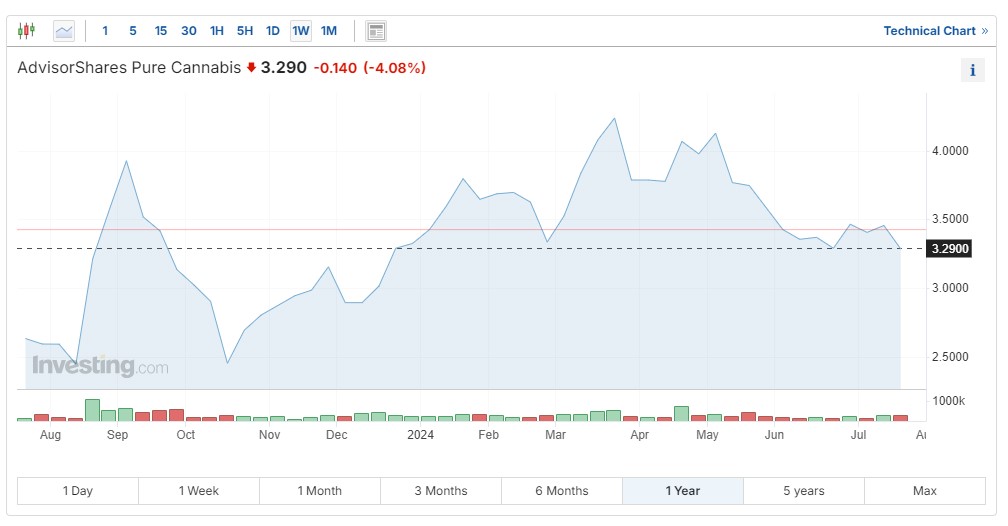
Of course, investors are always advised to do their due diligence before investing in any stock, ETF or industry.








