4 Psychedelic Stocks to Consider as Clinical Trials Heat up
2024.08.06 18:08
Psychedelic stocks have captured the spotlight on Wall Street as promising new treatments for conditions with significant unmet medical needs make their way through clinical trials. Amid all the investor excitement, the Psychedelic Invest Index, which tracks several psychedelic stocks, is up from 20.84 to 27.05 year to date.
Several biotechnology firms have treatments in development. While biotech stocks can be volatile, investors who look carefully may be able to benefit from some of these potential opportunities. In fact, a recently published survey shows that most investors actually favor psychedelic stocks, making this roundup particularly timely.
Most investors are in favor of psychedelic stocks
The survey entitled “Investor Insights into Psychedelic Stocks” conducted by Quantum Research Group, LLC found that 66% of investors consider themselves psychedelic friendly. Additionally, 66% of psychedelic-friendly investors plan to increase their holdings in psychedelic stocks in the next 12 months.
Psychedelic-friendly investors are those who already invest in these stocks or who are considering investing in them in the future. The biggest draw for psychedelic investors to psychedelic stocks was growth potential, as 73% listed this factor as their top reason for interest in the sector.
Expectations for high financial returns on psychedelic stocks ranked as the second-most-popular factor at 70%. Other factors drawing psychedelic-friendly investors to the space include advancements in research in the sector (67%) and optimism about potential market or sales growth (57%).
Now without further ado, here are four psychedelic stocks that have displayed promising results on their clinical trials so far.
ATAI Life Sciences
ATAI Life Sciences BV (NASDAQ:) (ATAI) has strategic investments in a number of psychedelic treatments, including one it’s developing with Compass Pathways, next on this stock list. Most recently, ATAI announced an update on Beckley Psytech’s Phase 1/2a trial of ELE-101, a form of intravenous psilocin, for major depressive disorder.
Dosing for the next phase has begun following promising Phase 1 data that suggested ELE-101 induced high-intensity, short-duration psychedelic experiences, indicating a two-hour treatment window in the clinic. A two-hour in-clinic treatment paradigm has already been established by the approved drug Spravato.
ATAI also has some psychedelic treatments of its own in the works, albeit in earlier stages. VLS-01, a form of dimethyltryptamine (DMT), is in Phase 1 trials for treatment-resistant depression, while IBX-210 or Ibogaine is in Phase 1 for opioid use disorder. The first data on VLS-01 is expected in the second half of this year.

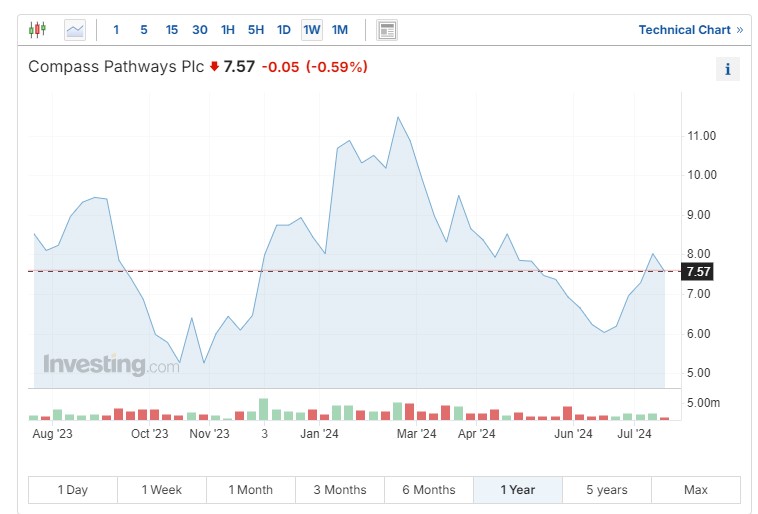
Compass Pathways
Compass Pathways Plc (NASDAQ:) (CMPS) is developing a psilocybin pipeline for mental health patients undergoing in-clinic therapy. The company has completed two of the largest studies on psilocybin and is conducting clinical trials for treatment-resistant depression, post-traumatic stress disorder and anorexia nervosa.
COMP 360, a form of psilocybin, is entering Phase 3 trials for depression and is currently in Phase 2 trials for PTSD and anorexia. In May, Compass announced a durable symptom improvement through 12 weeks in an open-label, Phase 2 trial of COMP 260 in PTSD. The company expects data from its Phase 3 trial in depression patients in the fourth quarter.
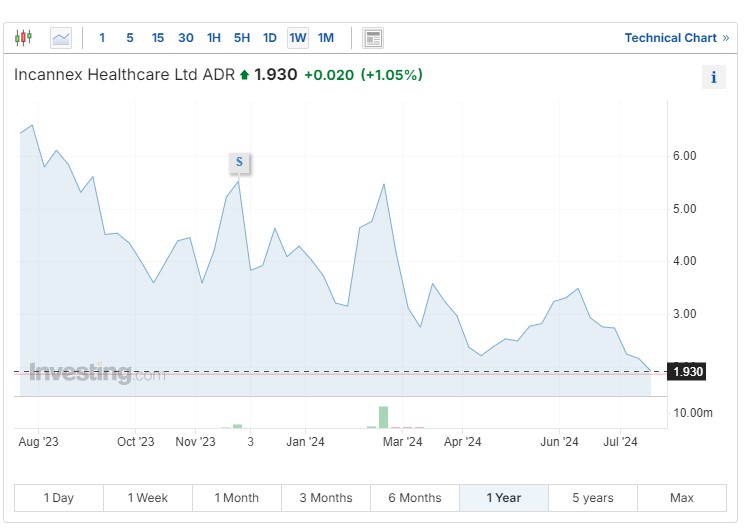
Incannex
Incannex Healthcare Ltd ADR (NASDAQ:) (IXHL) currently has three lead drug candidates and several secondary assets with established proofs of concept in the works. PSI-GAD is the company’s psilocybin-assisted psychotherapy treatment for generalized anxiety disorder.
PSI-GAD addresses unmet medical needs posed by antidepressant medications, which are currently the standard of care for generalized anxiety disorder. However, less than half of patients achieve remission on these medications, leaving the door open for additional treatment options.
In 2024, Incannex announced positive topline results from its Phase 2 trial of PSI-GAD1 for anxiety, reporting that the study achieved its primary endpoint with a statistically significant reduction of 12.8 points from the baseline on the Hamilton Anxiety Rating Scale.
It also represented a 9.2-point improvement over psychotherapy with placebo. Forty-four patients receiving psilocybin displayed at least a 50% reduction in their anxiety score, while 27% showed remission — more than five times the rate achieved with therapy and placebo.
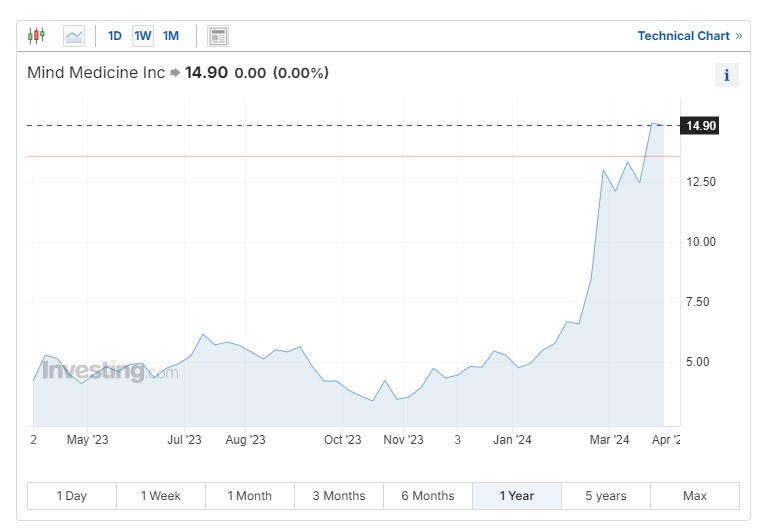
Mind Medicine
Mind Medicine Inc (NASDAQ:) (MNMD) is working on MM120 (lysergic acid diethylamide, better known as LSD, D-tartrate), an oral dissolving tablet designed to treat generalized anxiety disorder. The company is also working on MM402, a form of MDMA currently in pilot clinical trials for autism.
In June, the company announced a “constructive” end-of-Phase 2 meeting with the FDA for MM120. Both the company and the agency are aligned on the requirements for the Phase 3 clinical trial, which is scheduled to begin in the second half of this year.
The Phase 2b trial assessed four doses of MM120 and demonstrated “rapid, clinically meaningful and statistically significant improvements” on the Hamilton Anxiety rating scale at weeks four and 12, with a 65% response rate and a 48% clinical remission rate sustained through week 12.
Mind Medicine stock has soared 143% year to date, bringing its 12-month return into the green at 102%. Notably, MindMed was added to the and Russell 3000 indexes at the end of June, forcing all funds that track these indexes to purchase the stock. This addition has significantly contributed to the recent increase in its stock price.
Investing in psychedelic stocks
The favorable clinical trials announced by most of the companies on this list offer hope for patients with significant unmet medical needs. The treatment market for generalized anxiety disorder alone is expected to reach $8.1 billion by 2027, highlighting the immense need for effective treatments targeted by the companies above.
With the psychedelic market projected to be worth $2.7 billion in 2024 and surpass $7.35 billion by 2031, the sector looks poised for tremendous growth in treating such conditions. Finally, the recent investor survey demonstrates that most psychedelic-friendly investors are aware of this high expected growth potential, which could bode well for psychedelic stocks.
Of course, investors are always advised to do their own due diligence before investing in any stock, sector or idea.
Disclosure: Ari Zoldan is the CEO of Quantum Media Group, and Incannex is a client of Quantum Media Group.








